Stem cells create new heart cells in baby mice, but not in adults, study shows
By Krishna Ramanujan
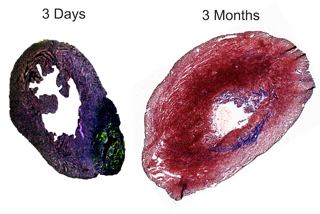
In a two-day-old mouse, a heart attack causes active stem cells to grow new heart cells; a few months later, the heart is mostly repaired. But in an adult mouse, recovery from such an attack leads to classic after-effects: scar tissue, permanent loss of function and life-threatening arrhythmias.
A new study by Cornell and University of Bonn researchers found that stem cells did not create new heart cells in adult mice after a heart attack, settling a decades-old controversy about whether stem cells play a role in the recovery of the adult mammalian heart following infarction -- the leading cause of sudden death in the developed world -- where heart tissue dies due to artery blockage.
"If you did have fully capable stem cells in adults, why are there no new heart cells after an infarct? And is this due to the lack of stem cells or due to something special about the infarct that inhibits stem cells from forming new heart cells?" asked Michael Kotlikoff, the Austin O. Hooey Dean of Cornell's College of Veterinary Medicine, and senior author of the paper appearing Aug. 29 in the Proceedings of the National Academy of Sciences.
Co-author Michelle Steffey, a small-animal surgeon in Cornell's veterinary college, developed a procedure to infarct a neonatal mouse heart that is only one-tenth-of-an-inch wide. "It was a tour-de-force technically to infarct and recover those baby mice," said Kotlikoff.
The baby mice grew new heart cells and almost completely recovered from infarction, proving that the infarction did not inhibit stem cells from growing new heart cells. The same procedure was carried out on adult mice and no new heart cells formed, confirming that adults do not have the requisite stem cells to create new heart cells, called myocytes, though new blood vessel cells were created.
To track the stem cells, Kotlikoff and colleagues used a mouse model they developed in which cells fluoresce green when the stem cell marker c-kit is present. In the experiment, after infarction, cells with the c-kit marker fluoresced green in neonatal and adult mice.
"In looking at the adult responses, we were able to prove that the c-kit-marked cells do not form heart cells, but form all of the new blood vessels within the infarct," said Kotlikoff. The stem cells found in the adult heart "have lost the ability to become heart cells," he said. It is known that developmentally single stem cells differentiate into all tissues at the start of life, but over time these cells become "developmentally restricted" or specialized to form only certain tissues, he added.
The study also showed for the first time that vessel stem cells in the adult heart originate there and are not recruited from bone marrow, as has been reported. Those reports have justified a controversial procedure in which bone marrow cells are injected into patients with infarctions.
Finally, the study settles the question of whether new heart cells in a neonatal mouse come from undifferentiated stem cells or from pre-existing heart cells that divide. To answer the question, the researchers used another mouse model where heart cells fluoresced red and undifferentiated stem cells fluoresced green. These two cell types were separated. The researchers found that the green stem cells that had moved into the infarct formed beating red heart cells in culture, proving that the stem cells had become heart cells.
Sophie Jesty, an associate professor and resident in cardiology at Cornell's College of Veterinary Medicine, is the paper's lead author. Researchers at the University of Bonn analyzed the mice to understand and quantify new myocyte formation.
The study was funded by the National Institutes of Health, New York State Stem Cell Science and the European Union Seventh Framework Programme.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe