Imaging Facility adds two tools for microscopy
By Krishna Ramanujan
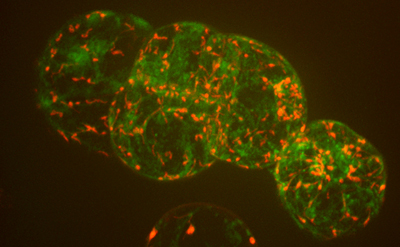
Cornell’s Imaging Facility owns microscopes, scanners and ultrasound units for revealing details that can’t be seen with the naked eye. Now the facility has added two more state-of-the-art machines, one to extract tiny samples for genetic analysis and another to image fast microscopic events.
The Imaging Facility, located in Weill Hall and in the College of Veterinary Medicine, specializes in microscopy, whole organism imaging and high-resolution micro-CT (computed tomography), for visualizing samples from cells and engineered tissues to fossils, tomatoes and sharks.
The facility now has a spinning disk confocal microscope that enables users to image and manipulate fluorescent specimens rapidly; and an instrument for laser capture microdissection, which allows researchers to isolate specific cells or tissues from a sample by slicing out particular regions with a laser.
Once cells or tissues are cut, the laser capture microdissection machine pops the new sample into a tube where its genomic or proteomic profile can be analyzed. Normally, a sample with many tissue types is ground up and genetically sequenced. Researchers must then piece together fragments of genetic code from the potpourri. This new method allows researchers to isolate specific cells or regions prior to genetic analysis.
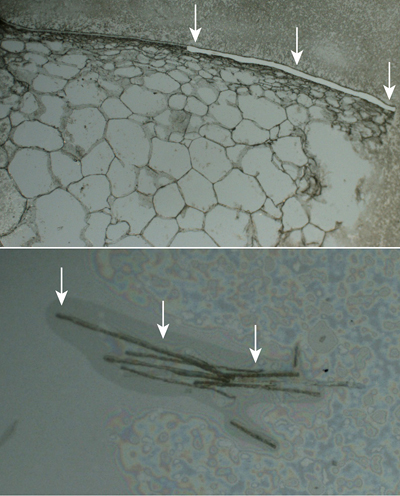
Cornell has two other laser capture microdissection tools on campus. The new one in the Imaging Facility is part of the Biotechnology Resource Center (BRC), which provides Cornell researchers with an array of state-of-the-art instruments and services. Managed by Cornell’s Institute for Biotechnology, whose director is Jocelyn Rose, professor of plant biology, the BRC comprises seven facility laboratories focused on genomics (DNA sequencing, genotyping and microarrays), epigenomics, proteomics and mass spectrometry, bio-IT, bioinformatics and computational biology, advanced technology assessment and imaging. These tools and services are available to the university community and to investigators at other institutions.
The laser capture microdissection unit provides another capability for genomic analysis that can be bridged with other BRC resources for every phase of an experiment, said research scientist Rebecca Williams, the Imaging Facility’s director.
“A researcher can microdissect a sample and end up with a tube of cells. Then we can help them find the correct genomics core facility” to have that sample sequenced, and then put them in touch with a biostatistics technician who can help analyze the sample, she added.
By raising and lowering the field of view, the new spinning disk confocal microscope can take repeated shots that are stitched together to create 3-D images. It can shoot more than 100 frames per second, allowing researchers to record such quick processes as calcium signaling or other fast movements. It also allows researchers to use photoactivated proteins (that turn on or change colors when hit with light) or photobleached proteins (that turn off when hit with light), providing insights to how proteins move within cells.
“Spinning disk confocal microscopes have been around for maybe 10 years, but this system is fully loaded; it’s a fancy system,” said Williams. It is fitted with three cameras for 3-D imaging of various colors, a photomanipulation unit, and control of such environmental conditions as carbon dioxide and oxygen levels.
Along with image analysis and visualization, the facility has software to manage the amount of data generated from taking 100 images per second, for example.
“Our job is to train people in these technologies and make it as easy as possible to use them,” Williams said. “We train 100 researchers a year to use these microscopes.”
The BRC was previously known as the Cornell University Life Sciences Core Laboratories Center.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe