A way to measure the bonds that hold together a single molecule is developed by Cornell physicists
By Bill Steele
Ever since the invention in 1982 of the scanning tunneling microscope (STM), which can see single atoms, scientists have been trying to use the instrument to examine the bonds that hold atoms together in molecules.
In a significant advance, a team of Cornell University physicists has successfully made a measurement of the frequency at which atoms in a bond are vibrating against each other in a single molecule of acetylene. The research for the first time provides a way to identify single molecules by their vibrational signatures and to study how their bonds change during chemical processes. It could lead to better understanding of how catalysts work and a new way to study biological molecules like DNA.
The experiment by Cornell physics graduate students Barry Stipe and Mohammad Rezaei and Wilson Ho, professor of physics, is described in the June 12, issue of Science.
Vibrational spectroscopy is the study of the energy (which for scientists identifies the frequency) of vibration of molecular bonds. Atoms are held together in molecules because the negatively charged electrons in one atom are pulled toward the positively charged nucleus of another and vice versa. But at the same time, the electrons in one atom repel those in the other, and the protons in the nuclei do the same.
This constant push-pull can create a vibration, as if the atoms were connected by tiny springs in constant motion. The vibration is unique for each possible arrangement of atoms in a molecule, and in the language of quantum mechanics, each vibration has a characteristic "energy level." An electron whose energy matches or exceeds that of a vibrational energy level in a molecule can pass through the molecule more easily.
By measuring current flow through molecules over a wide range of energies, scientists can create a "vibrational spectrum" that provides a fingerprint of the bonds in a molecule and thus is a powerful analytical method for identification of unknown chemicals.
For years the only useful way to do this type of vibrational spectroscopy was to pass a current through a thin film of a substance and vary the voltage, and therefore the energy of the electrons in the current. This method, called "inelastic electron tunneling spectroscopy," only gives an average reading on billions of molecules. Since the invention of the STM, scientists have searched for a way to do the same measurements on one molecule at a time.
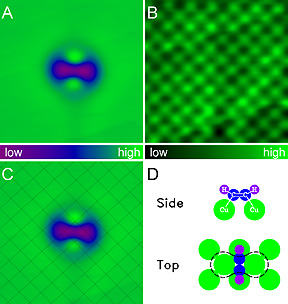
Stipe, Rezaei and Ho succeeded by using a specially made STM cooled to 8 degrees Kelvin (minus 265 degrees Celsius, minus 445 degrees Fahrenheit) to minimize molecular motion, and working with acetylene (C2H2) molecules bonded to a copper surface.
An STM consists of a needle so sharp that its tip can narrow to just one atom, suspended less than a billionth of a meter above the surface to be scanned. When a voltage is applied between the needle and the surface a tiny electric current, called a "tunneling current," flows between the two. To form an image, the tip is moved back and forth across the surface and its height is adjusted so that the current remains constant. A computer converts these movements into an image that shows individual atoms as "bumps" or "dips."
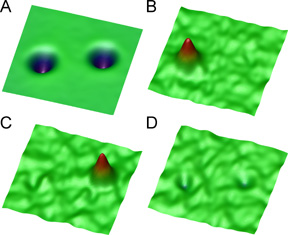
For these experiments the tip was held still at a constant height above a molecule of acetylene, and the voltage was varied between 0 and 500 millivolts (mV). A peak was seen at 358 mV, representing a point at which the energy of the electrons matched that of the vibration of the bond between a carbon atom and a hydrogen atom in the acetylene molecule.
Previous researchers had not been able to obtain such a clear result, the Cornell researchers say, because the change in current at the peak was less than variations caused by unsteadiness of the STM needle. The instrument the Cornell researchers built in their laboratory is capable of holding steady the height of its needle tip to better than 0.01 Angstroms, or less than 1 percent of the diameter of an atom. The precision is obtained by a combination of careful design and special electronics and software to control the instrument.
The ability to identify molecular bonds in a single molecule opens a new way of using the STM that the Cornell researchers call "vibrational microscopy." If a surface is scanned with the STM tip held at a constant current while the voltage is set to detect a particular molecular bond, molecules with that bond will be highlighted on the scan while other molecules will remain invisible.
The Cornell researchers demonstrated this by comparing the images of an ordinary acetylene molecule with that of a molecule in which both hydrogen atoms are replaced with deuterium, or "heavy hydrogen." At 358 mV the scan shows only the ordinary acetylene molecule, while at 266 mV it shows only the molecule of deuterated acetylene. By increasing the resolution, the researchers say, the technique they have developed can, in principle, be used to image the positions of each of the chemical bonds within a single molecule.
The title of the paper in Science is "Single Molecule Vibrational Spectroscopy and Microscopy." The research was supported by a grant from the National Science Foundation.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe