Cornell researchers to kick-start fuel cell development with $2.25 million federal award to find new materials for cheap, efficient technology
By David Brand
The U.S. Department of Energy (DOE) has awarded Cornell University $2.25 million over three years to establish the Cornell Fuel Cell Institute (CFCI). The institute will research new materials to kick-start the development of fuel cells that would be both efficient and cheap to produce.
The new approach to the electrochemical device, that in its traditional form converts hydrogen and oxygen into water and produces electricity and heat in the process, aims to make a significant improvement in the technology by discovering and exploiting new materials based on recent discoveries in Cornell laboratories. Indeed, some of the possible fuel cell technologies that could result from the research might not even involve hydrogen as a fuel.
"This is an interdisciplinary approach to good science with an obvious technological import," says Cornell chemist Francis (Frank) DiSalvo, one of the program's two principal investigators. "It is not often you can see such a close link between the basic research and a potential payoff." DiSalvo is director of the National Science Foundation (NSF)-funded Cornell Center for Materials Research, which manages a group of shared experimental facilities that will provide many of the analytical tools for the fuel cell research.
Some of the research also will take place in two other NSF-funded centers, the Cornell Nanoscale Facility and the Cornell High Energy Synchrotron Source.
Although the CFCI initially will involve just six Cornell researchers and one from the California Institute of Technology, ultimately it could call on the expertise of many of the 100 faculty members involved in materials research at Cornell. The DOE funds primarily will support graduate and postdoctoral research.
DiSalvo's co-principal investigator and CFCI director, Héctor (Tito) Abruña, states his conviction that despite the fuel cell's long-heralded promise, the last decade of intensive engineering is nearing limits that can only be overcome with the development of new materials. "In the past 20 years, there has been little materials research aimed at improving fuel cells," says Abruña, who is professor of chemistry and chemical biology. "Most of the limits that current fuel cells face are in the materials themselves." Adds DiSalvo, "We want to make the materials effective and dirt cheap."
Despite the fact that fuel cell technology has been available for decades, automakers are still a long way from making an affordable, durable and efficient fuel. General Motors, Toyota and Honda all have ambitious fuel cell programs, but they are at least a decade away from putting the technology into production, the Cornell researchers say.
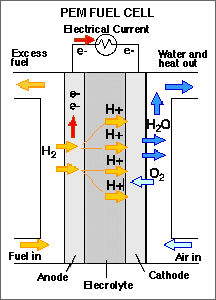
The most promising technology at present is the polymer electrolyte membrane fuel cell, which uses hydrogen gas as a fuel. One challenge in implementing fuel cell technology is the need for more efficient hydrogen generation, since the gas is not available as a resource in its pure state but must be obtained largely from water or hydrocarbons. Hydrogen gas also is difficult to store and distribute.
One potential solution is to attach the fuel cell to a costly device called a reformer, which turns hydrocarbon fuels into hydrogen. But, Abruña points out, it would be more efficient to bypass hydrogen and make direct use of the hydrocarbon or other liquid fuels, such as methanol and ethanol. One long-range CFCI goal is to make it possible to use a variety of fuels that also would provide a steppingstone to an eventual hydrogen economy.
"We now have anodes that work pretty well with methanol, which is converted at the anode into carbon dioxide and protons. Instead of throwing away the carbon dioxide, as would happen if a reformer is used, such a fuel cell could produce extra energy from the carbon and so produce a more efficient cell with less carbon dioxide output," says Abruña. (Fuel cells have two electrodes: the anode, which is the negative post, and the cathode, the positive post.)
CFCI has its origins in the research of Cornell doctoral candidate Sean Smith who, working with Abruña, found that single-crystal platinum, modified by depositing just a few atoms of bismuth on its surface, is much better at oxidizing the simplest fuel --- formic acid (the same chemical used by ants) -- than is platinum on its own. Curiously Sir William Grove, who invented the hydrogen fuel cell in 1839, also used platinum metal as fuel cell electrodes. However, when other fuels are used, platinum loses much of its ability to promote the fuel cell reactions.
Single crystals of platinum can be cut in different ways to expose various arrangements of atoms at the surface, each arrangement enabling a different activity. But fuels other than pure
hydrogen produce carbon monoxide, which "poisons" the fuel cell by strongly binding to the surface of the platinum and resulting in dramatic losses in efficiency. Adding ruthenium to the platinum mitigates the poisoning, and the addition of a few atoms of bismuth "mitigates it by an enormous amount," Abruña says.
Indeed, DiSalvo and his colleagues found that a compound of platinum and bismuth is an ideal fuel cell material. It is not an alloy like stainless steel or the platinum-ruthenium that many fuel cells use, but a so-called ordered intermetallic compound in which atoms are very specifically arranged. "These ordered intermetallic compounds have not been explored for use in fuel cells, so even though platinum-bismuth is quite good, we are searching for other such compounds that should do even better," DiSalvo says. "The team has found several other compounds that also are promising, and we expect that in the near future our rate of searching will increase perhaps a thousandfold."
Bruce Van Dover, a Cornell professor of materials science and engineering who is an expert in combinatorial methods (techniques for making and testing many complex metal materials in parallel), will search through thousands of intermetallic compounds to find compositions and structures that are even more attractive as fuel cell electrodes.
Other CFCI researchers will look at new synthetic materials for the fuel cell's two electrodes and the electrolyte, or membrane, that is placed between them to conduct positively charged ions. In the materials science and engineering department, Associate Professor Ulrich Wiesner will look at "one pot" synthetics, such as flexible ceramics, and Professor Emmanuel Giannelis will investigate composites made from clays and polymers.
The materials produced by the group will be examined both at Cornell and at collaborating companies, including MTI Micro Fuel Cells in Albany, N.Y., which is planning to market a fuel cell that uses methanol as a fuel. In May the company demonstrated a methanol "battery" powering a combination mobile phone, personal digital assistant and a digital camera device. CFCI also is discussing partnerships with General Motors Fuel Cell Division, Corning and Exxon Research.
The Cornell researchers also plan to work with methanol as a fuel but are exploring other possibilities, such as ethanol -- obtained from biomass -- or other hydrocarbon sources. "Intermetallic compounds in our view have the potential to use ethanol," says Abruña. Barry Carpenter, Cornell professor of chemistry and chemical biology, is working with the group to identify other potential fuels that might be used efficiently in fuel cells and with different electrode materials.
CFCI is not choosing a specific technology in its research. Says DiSalvo, "We are not choosing a winner, we are just exploring new materials and what we find might then help decide which technology is going to be the right one.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe