Model recreates wear and tear of osteoarthritis
By Anne Ju
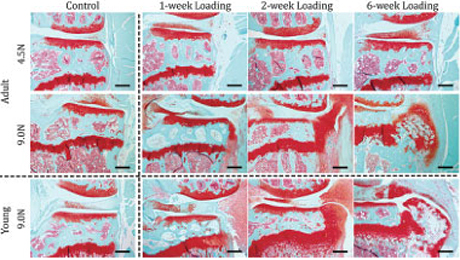
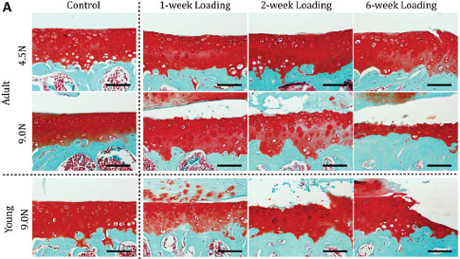
There’s a reason osteoarthritis is often called wear-and-tear arthritis: Repeated stress on joints over time results in degeneration of the soft cartilage that normally distributes loads to the joints.
Recreating how joints bear stress could lead to a better understanding of the mechanical and physiological processes involved in the development of osteoarthritis. Cornell engineers have created a loading model that simulates prolonged joint loading, leading to similar conditions found in osteoarthritis sufferers. The model highlights not only cartilage degeneration and stiffening of bone, but also related changes immediately adjacent to the joint.
The study was published in the journal Arthritis and Rheumatism this month (Vol. 65 No. 6). Lead author Frank Ko is a graduate student in the research group of senior author Marjolein van der Meulen, the Swanson Professor of Biomedical Engineering in the Sibley School of Mechanical and Aerospace Engineering. The collaborative work included co-authors Steven Goldring, Timothy Wright and Mary Goldring of the Hospital for Special Surgery in New York City, with which Cornell has a longstanding collaboration in orthopedic biomechanics research.
“This research project represents a unique collaboration between biomedical engineers and cell and molecular biologists at our two institutions,” Steven Goldring said. “Importantly, the findings can be directly translated into therapeutic interventions that potentially can prevent the initiation and slow the progression of osteoarthritis.”
The model was developed by the van der Meulen group previously to simulate bone changes with loading. Based on these data and in collaboration with the Hospital for Special Surgery, a hypothesis emerged that changes in bone stiffness with loading might be driving cartilage changes, leading to its degeneration and the development of osteoarthritis.
“We had never looked at what’s going on in cartilage; our sole focus was bone,” van der Meulen said.
Their model applied controlled loading to the mouse knee. In the study, different load magnitudes were applied to the knees of growing and adult mice over 1-, 2- and 6-week periods. The method is noninvasive, making it a more reliable representation of the development of osteoarthritis due to loading than surgically induced experimental changes to the joints, like removing a meniscus or cutting ligaments.
They found that the articular cartilage located on the tibia developed damage that increased with severity and duration of loading in the knee joints of mice. Both young and old joints developed bone spurs, or periarticular osteophytes, which are outgrowths of bone at the edge of the joint associated with heavy and repeated loading.
The literature suggests that loading at moderate levels can stimulate cartilage growth, whereas these data demonstrate that more severe loading definitely creates joint damage, Ko said. That might be why individuals who exercise reap the benefits of healthier cartilage, but too much exercise causes damage. This model allows this question to be examined further.
The study was a collaboration between engineers, clinicians and biologists who collectively provide basic science expertise in bone and cartilage and applied perspectives to translate the findings to the clinic – a collaboration that makes sense given the poorly understood, but hypothesized relationship between changes in bone and changes in cartilage that result in osteoarthritis, van der Meulen said.
By reliably recapitulating the key features of osteoarthritis, the model could pave the way for future experiments focusing on other potential contributing factors to the disease, as well as treatments to inhibit disease progression.
The study, ”In vivo cyclic compression causes cartilage damage and subchondral bone changes in mouse tibiae,” was supported by the National Institute of Aging and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe